Raman Spectroscopy:
The Indian scientist named after Sir C.V.Raman discovered the scattering of blue light in the ocean. He won the nobel prize in 1930, accomplished using filtered light as a monochromatic source of photons, a coloured filter as a monochromator, and a human eye as a detector. The technique became widely used after the invention of the laser.
Introduction:
When the sample is introduced into intense beam of monochromatic light, the scattered light is recorded by using the detector and the signals gets digitalized.
Raman spectroscopy determines the vibrational and rotational level spacings from the energy shifts of scattered light.
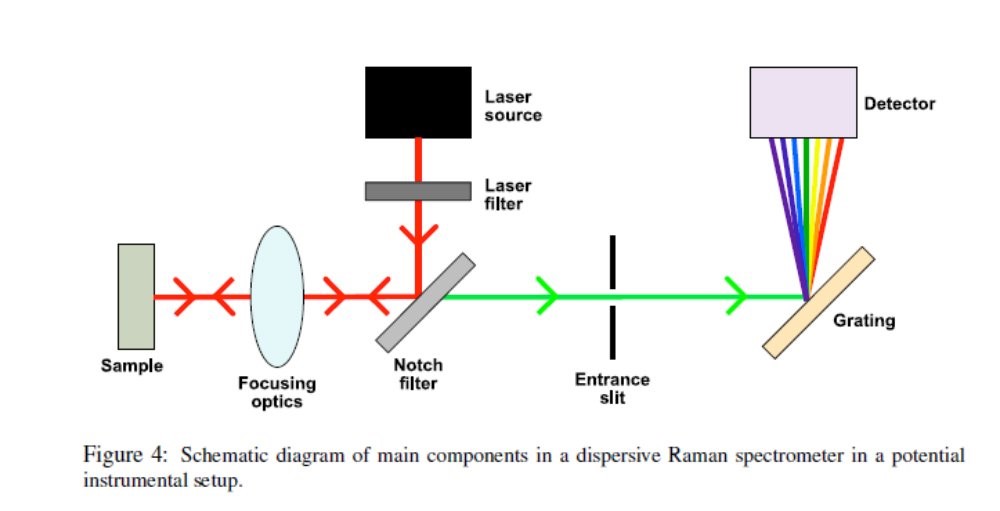
Principle:
If the molecule have no permanent dipole moment would not give pure rotational spectra. If the molecule does not have any change in dipole moment it does not give vibrational spectra. But in Raman spectroscopy, we can determine rotational and vibrational level spacings of such system. We can determine the bond lengths and force constants of such molecules.
For example., O2, N2, H2.
Types of scattering:
The photons can be scattered by
- Elastic scattering
- Inelastic scattering

In elastic scattering, the energy of incident photon is same as that of the scattered photon and gives rise to Rayleigh scattering.
In inelastic scattering, the energy of scattered photon is greater than or less than the incident photon and gives rise to Raman scattering.
If the energy of scattered photon is less than the energy of incident photon then it is called Stoke’s line.
If the energy of scattered photon is greater than the energy of incident photon then it is called Anti stoke’s line.
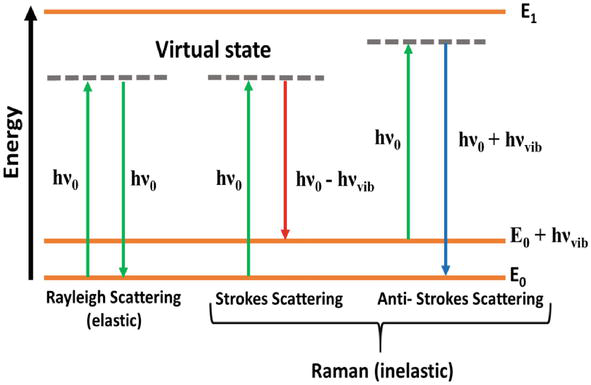
Condition:
If the molecule has a centre of symmetry then it is Raman active. This is known as rule of mutual exclusion.
Overtone:
In vibrational spectroscopy, the transition occurs from ground state(v=0) to second excited state(v =2) then it give rise to overtone.
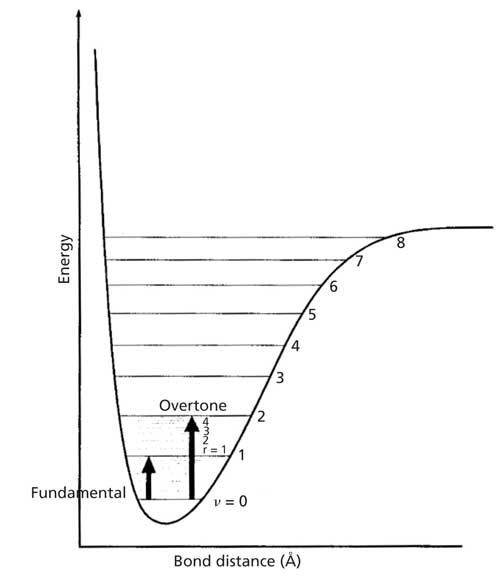
Hot band:
Hot band is nothing but the transition between two excited states which neither occurs in the ground state. It is observed when the molecule which is already in the excited state gets further excited.
Application of Raman Spectroscopy:
- Used to identify the inorganic compounds even in aqueous solution.
- Detection of molecular impurities and additives.
- Used to identify biological samples especially protein conformation.
- Only small amount of sample is needed for analysis.
Video: