Infra-Red Spectroscopy
Introduction:
Infra-Red Spectroscopy gives sufficient information about the structure of the unknown compound. The absorption of infra red radiation causes various bands for various molecules. The ordinary infra red region extends from 2.5µ to 15 µ. The region below the lower region that is from 0.8 µ to 2.5 µ is called as near Infra-red and the region which beyond 15 µ that is from 15 µ to 200 µ is called as Far Infra-red region.
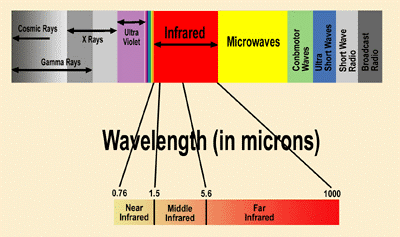
The absorption of radiations can be expressed in terms of wavelength or wave number.
Wave number = 1/ wavelength in centimeters.
Principle:
It mainly works on the principle of change in dipole moment of the molecule. Not all the bonds in the molecule absorbs infra red radiations, only the bond which is having change in dipole moment absorbs infra red radiations. The absorption of radiation causes excitation of the molecule from lower vibrational level to higher vibrational level. That vibration is also associated with number of rotational levels. So Infra-Red Spectra is also called as Vibrational-rotational spectra.
It gives information about
- Mass of atoms in the molecule
- Strength of bonds
- Arrangement of atoms in the molecule
No two compounds have the similar infra red spectra except the enantiomers.
When it absorbs the radiation the distance between the two atoms increases or decreases but the atoms remain in the same bond axis and gives rise to
- Stretching
- Bending

Stretching:
In this, the bond length increases and decreases with respect to the absorption of radiation. There are two types of stretching vibrations.
Symmetric stretching:-
In this type, the movement of atoms are in the same direction.
Asymmetric stretching:-
In this vibration, one atom moves to the central atom and the another one moves away from the central atom.
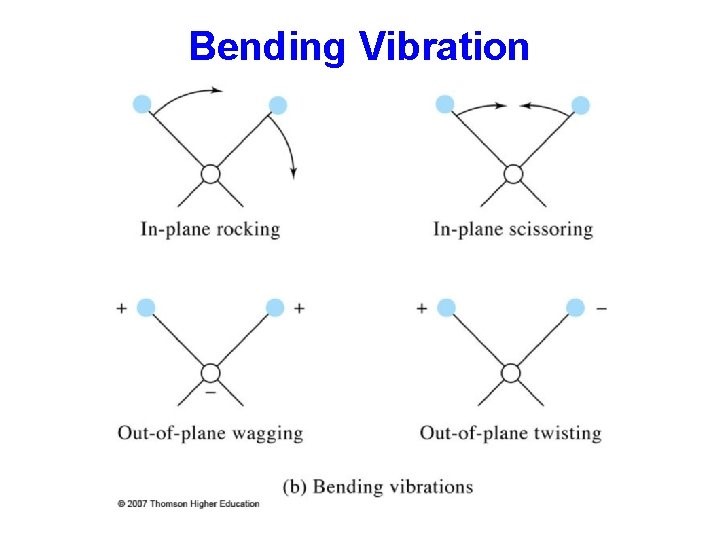
Bending:
Bending are of four types.
Scissoring:-
In this type, two atoms approach each other.
Rocking:-
Movement of the atoms takes place in the same direction.
Wagging:-
Two atoms move up and down the plane.
Twisting:-
One atom moves up the plane another one moves down the plane.
Finger print region:
The region below 1500cm-1 is known as finger print region. It is used to compare and also for the detection of certain functional groups like esters, ethers etc.,
Factors influencing Infra Red spectra
- i) Coupled vibrations and Fermi resonance:
If the molecule undergoes both symmetric and asymmetric stretching then it is called coupled vibration. Usually asymmetric stretching is having higher frequency than the symmetric stretching.
A type of resonance occurs in the case of coupled pendulum is called Fermi resonance.
- ii) Absorption are concentration dependent.
iii) Effect of hydrogen bonding:
The presence of hydrogen bonding increases the absorption frequency.