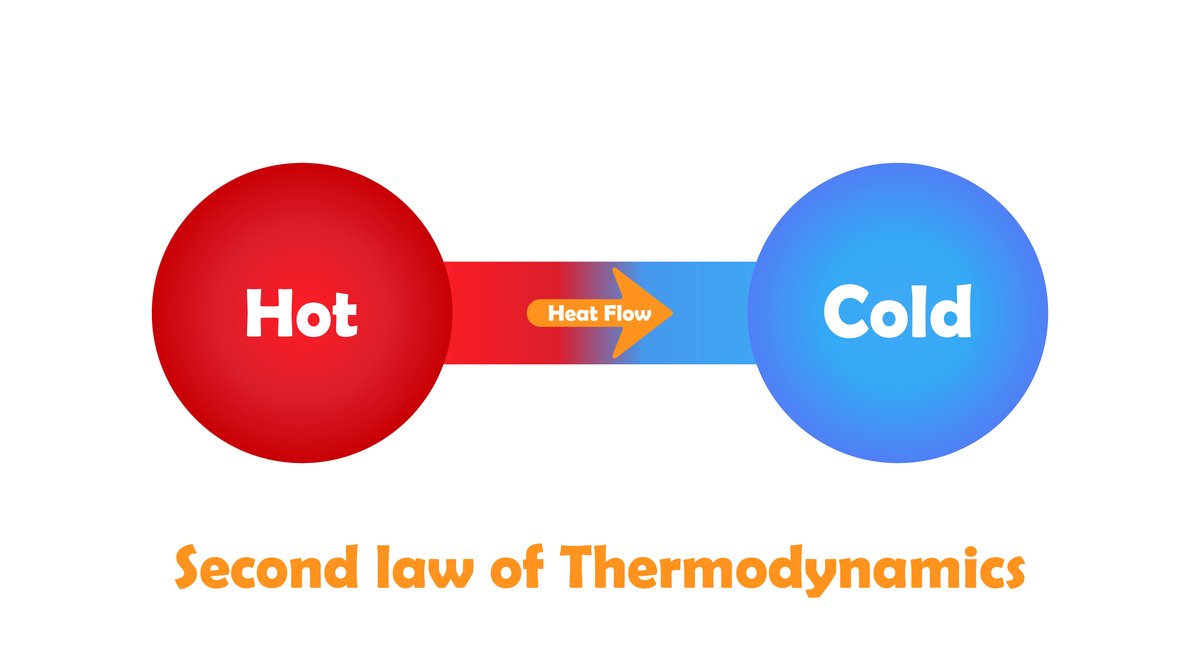
Second law of Thermodynamics:
The second law of thermodynamics introduces another state function called entropy. Entropy is a measure of the molecular disorder (randomness) of a system.
It is defined as, dS = dqrev /T
The SI unit of entropy is JK-1.
Kelvin Planck statement:
It is impossible to construct a machine that absorbs heat from a hot source and converts in to completely work by a cyclic process without transferring a part of heat to a cold sink. The second law of thermodynamics explains why even an ideal frictionless engine cannot give 100% efficiency.
Efficiency = work performed / heat absorbed
Clausius Statement:
It is impossible to transfer heat from a cold reservoir to a hot reservoir without doing some work.
Entropy change accompanying in the change of phase
When the substance undergoes change of state there is a change in entropy.
∆S = q rev / T = ∆H rev / T
Entropy of fusion:
The heat absorbed when one mole of a solid melts at its melting point reversibly is called molar heat of fusion.
∆Sfusion = ∆Hfusion / Tf
Where is the Tf melting point.
Entropy of vapourisation:
The heat absorbed when one mole of liquid boils at its boiling point reversibly is called molar heat of vapourisation.
∆Sv= ∆Hv / Tb
Where is the Tb is the boiling point.
Entropy of transition
The heat change when one mole of a solid changes reversibly from one allotropic form to another at its transition temperature is called enthalpy of transition.
∆St = ∆Ht/ Tt
Where is the Tt is the transition temperature.
Gibbs free energy:
Gibbs free energy is defined as
G = H – TS
It is an extensive property and it is a single valued state function.
Criteria for spontaneity of a process:
- If the enthalpy change of a process is negative, then it is exothermic and may be spontaneous.
- If the enthalpy change of a process is positive, then it is endothermic and may occur spontaneously.
- The Gibbs free energy which is the combination of the above two should be negative for a reaction to occur spontaneously, i.e. the necessary condition for a reaction to be spontaneous is ∆H – T∆S<0.