What is Copper sulphate?
Cubric sulphate is commonly referred to as peacock sulfate and copper sulphate. The chemical formula of kubric sulphate is CuSO4. Copper sulphate is found in blue. Copper sulphate is water soluble. Its color is blue due to the presence of copper.

Formula CuSO4
IUPAC ID Copper(II) sulfate
Molar mass 159.609 g/mol
Melting point 110 °C
Density 3.6 g/cm³
Soluble in Water
Copper Sulphate Formula:
Copper react with sulphte to form copper sulphate reaction.
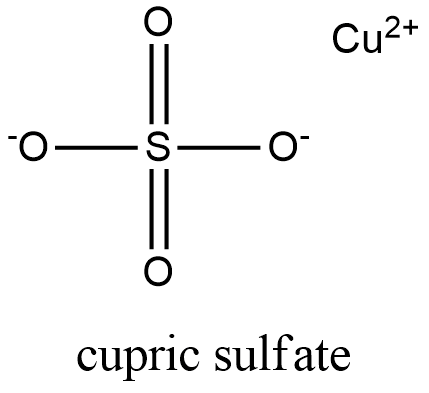
Structure

Balancing Equation Copper Sulphate:
Example
Copper sulphate reacts with sodium hydroxide to form the coper hydroxide and the sodium sulphate. As usual it will be double decomposition and split into cu 2+ and SO4 2. Sodium hydroxide then decomposes into Na + and OH. After that two ions will join.
CuSO4 + 2NaOH → Cu(OH)2 + Na2SO4
Uses of Copper Sulphate:
- Copper is essential for the blood’s need. If I soak it for a few hours and drink it, the body organs will work very well.
- Copper sulphate is used for public health and safety.
- Copper sulphate is used as a fungicide, herbicide and herbicide.
Side effects of copper sulphate:
- Inflammation of the upper part of the body.
- Is the absence of energy in our body.
- All the changes that take place in our body are seen differently.
- Disorders such as diarrhea and light sensitivity may occur.




















